Just last week on Nov 9, Pfizer made known that its experimental COVID-19 vaccine was more than 90 per cent effective. Pfizer and German partner BioNTech are the first drugmakers to release such data. But fast forward to today, we have a new player in town and the name to note is Moderna. According to the company, its experimental vaccine is 94.5 per cent effective in preventing COVID-19.
With so much hype surrounding this topic, here are 10 things you need to know about Pfizer’s and Moderna’s COVID-19 vaccines.
Pfizer’s COVID-19 Vaccine
Image Credits: Nikkei Asia
#1: More than 90 per cent effective
As we’ve briefly mentioned in the introduction, Pfizer’s vaccine is more than 90 per cent effective. This means that if 9 out of 10 people were to get the vaccine, they would attain immunity.
This is terrific news for many because previously, health experts have revealed that even a vaccine with an efficacy rating of between 60% to 70% would be a huge thing to rejoice for! Some governments were also ready to approve a vaccine if it only has a 50% efficacy rating.
But now, Pfizer is raising the stakes with a possible vaccine that could break the virus chain. In fact, Britain’s health minister Matt Hancock mentioned on Nov 16, “We’ll be ready to roll (the Pfizer’s COVID-19 vaccine) out as soon as it comes, we’ll be ready from the first of December…, but more likely is that we may be able to start rolling it out before Christmas.”
#2: Uncertainty on the period of effectiveness
While Pfizer’s vaccine is more than 90 per cent effective, no one knows how long it will be useful for. In other words, we have no idea how long the immunity gained from the vaccine can last us. It could be days, months, or years. Looking on the bright side, maybe it could be forever?
Another unknown factor is its successfulness on the profile of COVID-19 patients. There is little or no evidence that the vaccine works on COVID-19 severe cases, such as people who require hospitalisation for the virus.
#3: Vaccinated people may still carry the disease

Image Credits: LA Times
Talking about uncertainty, there’s the variability that vaccinated people could still carry SARS-CoV-2, the virus that causes COVID-19 and be infectious.
So even if one were to be injected with the vaccine and go about his or her day-to-day activities immune, he or she could still present a threat to others who aren’t vaccinated.
#4: Not a one-and-done deal
It will be delusional to think that it’s a one-shot all kill kind of super vaccine. A person will need at least two doses of the vaccine with a period of three weeks apart.
This could pose potential logistical and supply chain problems because supposedly everyone on earth requires that vaccine, then Pfizer will need to supply about 15 billion doses to meet the demand.
#5: Waiting time needed to get the vaccines
All good things must wait? Well, the economy is looking up for selected countries with the announcement of Pfizer’s initial trial results.
If approved, only 50 million doses would be available by the end of 2020. There’s also an estimate that only 1.3 billion quantities will be ready by the end of 2021. Mind you; there’s an estimate of 7.8 billion people in the world. With double doses needed, the supply vastly falls short.
Also, the need to store the vaccine at temperatures of -70°C or below poses a challenge for some countries, for example, in Asia, Africa, and Latin America who are already battling intense heat.
Moderna’s COVID-19 Vaccine
Image Credits: The Guardian
#6: Has a 94.5 per cent efficacy rate
Based on an interim data from a late-stage trial, Moderna said on Nov 16 that its experimental vaccine is 94.5 per cent effective in preventing COVID-19. This makes them the second US drugmaker to report such outstanding results.
But it’s important to note that the announced rating came after studying only 95 participants out of a total of 30,000 participants. While a little hope keeps the world going, it’s essential to wait for the updated results from the ongoing phase three trial. The outcome can overturn within a few days.
#7: At least two doses of the vaccine needed
If you’ve read the article in full, you would have known that Moderna’s vaccine is similar to Pfizer’s in terms of the doses needed. There will be a few weeks of waiting time required between the first and second shot.
A news report has it that after taking the second dose, a notable proportion of volunteers went through severe aches and pains. To be exact, 10 per cent experienced fatigue that interfered with their daily activities, while another 9 per cent had severe body aches. In response, Moderna feedback that the symptoms were short-lived.
#8: Questions about safety linger
Image Credits: BBC News
Similar to Pfizer’s, Moderna’s vaccine may have potential red flags. Apart from the side effects, as stated above, the public is not yet sure if there are other more severe consequences. For example, there is one possibility that it might cause cancer in the future.
BBC News reported that there are no significant safety concerns at the moment but highlights that nothing is 100% safe. But having said that, the public has nothing to worry about as Moderna can only apply for the US emergency use authorisation (EUA) after a two-month follow-up safety data.
#9: Does not require extreme cold storage
Compared to Pfizer’s COVID-19 vaccine which needs to thrive at extremely low temperatures, Moderna’s can be stored at just -20°C during shipping.
In terms of shipping logistics and distribution, Moderna’s vaccine is much more well-positioned. Once it reaches a distribution centre, the vaccine can be defrosted and stored for up to 30 days in a refrigerator at only 2°C to 8°C. Thus, overcoming one of the most significant limitations of Pfizer’s.
#10: Distribution may start as soon as next month
If the US Food and Drug Administration (FDA) grants Moderna EUA for the vaccine, Americans would probably be in line to get their first vaccination by December. At the time of writing, Pfizer has launched a pilot delivery programme for its experimental COVID-19 vaccine in New Mexico, Rhode Island, Tennessee and Texas.
However, even if Moderna has no green light, at least 20 million doses will still be available in December. This means that about 10 million people, most likely front-line healthcare workers, will receive the vaccine shots.
Vaccines at a glance
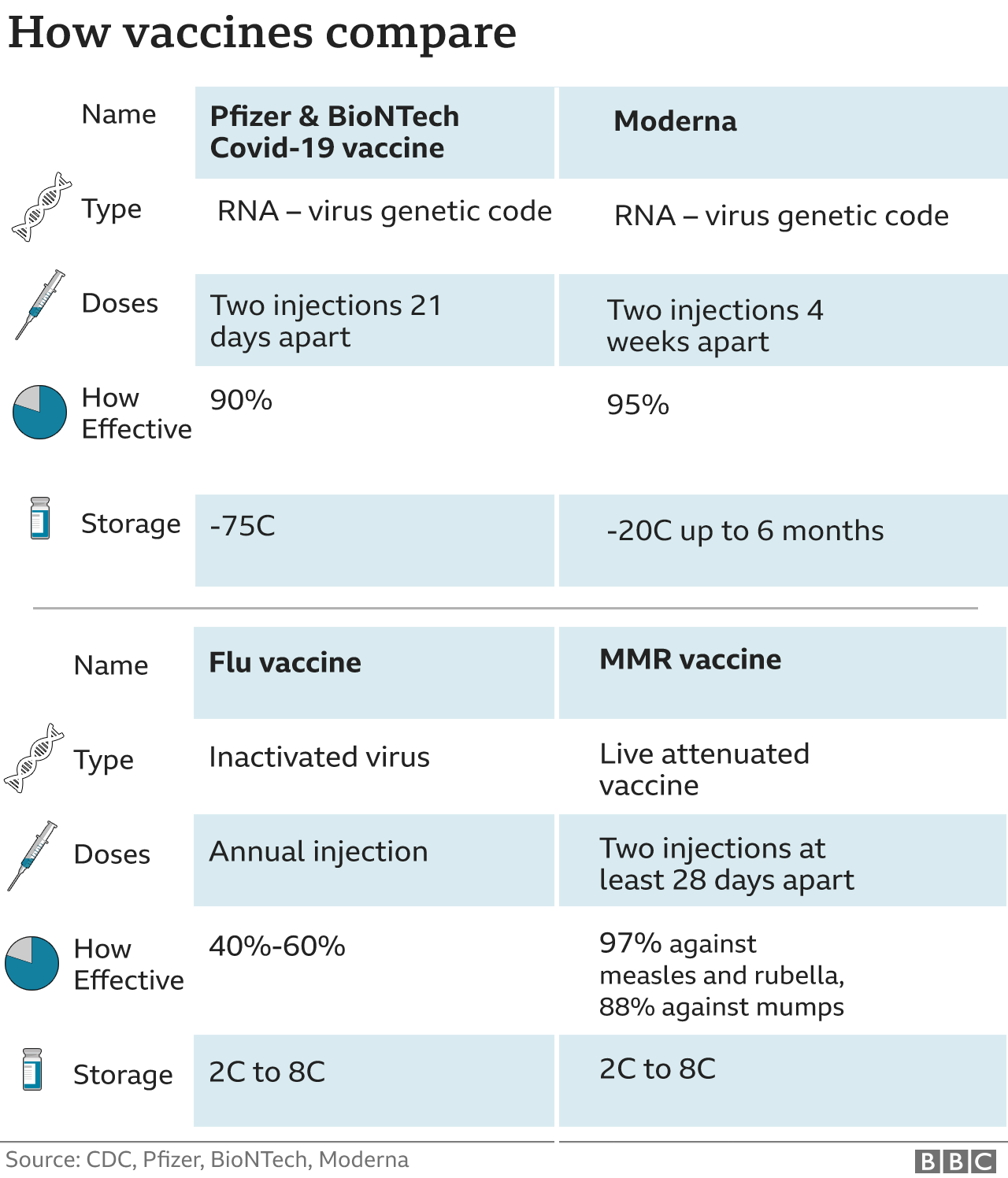
Image Credits: BBC News




